Selective depolymerisation of polyesters by earth-abundant iron catalyst
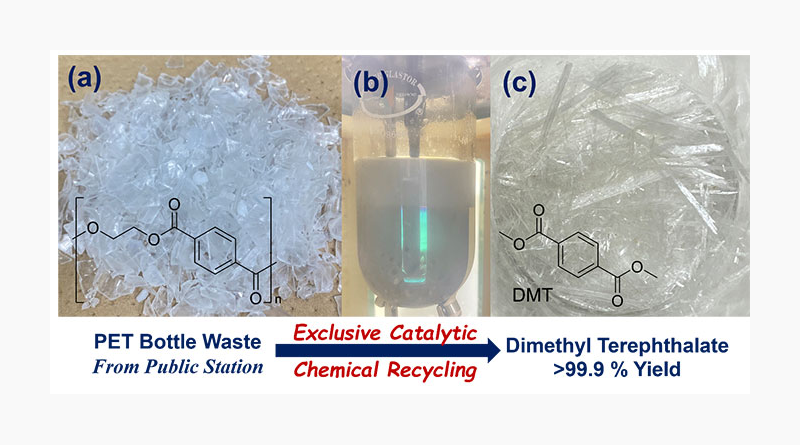
Fig. top: Perfect chemical conversion of PET waste bottles to raw chemicals (DMT). (a) PET bottle waste collected from the public station, (b) before reaction with methanol (30.0 g scale) in the presence of iron catalyst, (c) analytically pure product DMT collected by filtration (yield >99.9 %). © Kotohiro Nomura
Professor Kotohiro Nomura’s research group at Tokyo Metropolitan University has developed an efficient method for the exclusive depolymerization of PET, PET bottles and textile wastes, using alcohols and an inexpensive, readily available and earth-abundant iron catalyst. This method can provide a new possibility for the selective chemical conversion of polyesters.
Polyester is formed by repeated “ester bonds” created through the reaction of a carboxylic acid and an alcohol. PET bottles have traditionally been recycled through mechanical processes such as collection, sorting, and reprocessing. According to the researchers, these established methods have limitations that chemical recycling aims to address by breaking ester bonds and converting PET back into its chemical raw materials. Conventional chemical recycling approaches, however, often require high temperatures and significant amounts of acids and/or inorganic or organic bases. As a result, there is growing interest in developing simpler, more cost-effective, and environmentally considerate alternatives.
The research team has now developed a simple acid- and base-free method for quantitative chemical recycling of PET waste bottles, textile waste by depolymerisation with alcohol, using iron catalyst system producing the corresponding terephthalic acid diesters [dimethyl terephthalate (DMT), diethyl terephthalate (DET), bis(hydroxyethyl) terephthalate (BHET) etc.] exclusively (99.7 to 99.9 % yield) even under scale-up conditions. Addition of a minuscule amount of amine enhanced the catalytic activity without compromising selectivity. The catalyst system consisting of iron(III) chloride (FeCl3), which is inexpensive and widely available, and a certain amine demonstrated superior catalyst performance at 120-180 ºC. The method is also claimed to enable the selective depolymerisation of PET from a mixture of cotton and other plastics. This PET-focused chemical recycling approach is presented by the researchers as a promising solution for achieving a circular economy.
The research was conducted under the Japan Science and Technology Agency (JST) CREST program, Research Area “Precise Material Science for Degradation and Stability,” Research Theme “Development of Bio-Based Advanced Polymers and their Depolymerization, Chemical Recycle.”
The research paper is as follows. “Quantitative Chemical Conversion of PET Waste Bottles, Textile Wastes by Exclusive Transesterification with Alcohols by FeCl3–Amine Catalyst Systems,” ACS Sustainable Resource Management, November 12, 2025, doi: 10.1021/acssusresmgt.5c00447