Scientists transform plastic waste into efficient CO2 capture materials
The researcher team at the University of Copenhagen has developed a method in which decomposed PET plastic becomes the main ingredient in efficient and sustainable CO2 capture. It turns “one man’s trash into another man’s treasure”, they say, and go on to claim that their solution simultaneously addresses two of the world’s biggest challenges – plastic pollution and the climate crisis. These key global problems are often interconnected and, typically, the solution to one problem creates another one while the clock keeps ticking.
Photo top: Margarita Poderyte, lead author of the research paper (photo: Max Emil Madsen)
PET plastic is one of the most widely used types of plastic in the world but, when it has served its purpose, it becomes a pressing global environmental issue. In many parts of the world it ends up in landfills, where it breaks down into polluting microplastics that spread to the air, soil and groundwater and a large proportion also end up in the oceans.
“By turning waste into a raw material that can actively reduce greenhouse gases, we make an environmental issue part of the solution to the climate crisis,” says Margarita Poderyte from the Department of Chemistry at the University of Copenhagen, lead author of the research paper disclosing the invention.
The solution is a potential win-win on a global scale, where plastic waste not only does not end up in nature but also becomes an active player in climate mitigation.
BAETA: development and deployment

The new chemical technology enables PET plastic waste to be transformed into a primary resource, a new form of CO2 sorbent they have developed. The process ‘upcycles’ the waste to a new material named BAETA, which can absorb CO2 out of the atmosphere so efficiently that it easily compares with existing carbon capture technologies.
Over 60 per cent of PET (by weight) is carbon, which has an inherent chemical and physical ability to maintain the structure. This ability is enhanced by transforming the plastic by adding a quantity of ethylenediamine, a compound known for its ability to bind CO2.
The newly-developed Copenhagen University process breaks down the plastic from polymer to a monomer, giving the material, which the team has named BAETA, a chemical composition that is claimed to be effective in pulling CO2 out of the air and binding it.
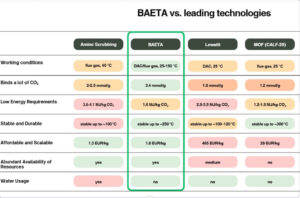
The BAETA material has a powdery structure that can be pelletised, and a chemically ‘upgraded’ surface, which enables it to effectively bind and chemically capture CO2. Once saturated, CO2 can be released through a heating process allowing the CO2 to be concentrated, collected and stored or converted into a sustainable resource.
Sustainable, flexible and scalable
In industrial plants, exhaust gases can be transmitted through BAETA units, which will cleanse them of CO2. When the BAETA material is saturated, its efficiency decreases; however, CO2 can be released from the plastic through a heating process, restoring its efficiency.
The carbon released can then be stored underground or used in, for example, Power2X plants, which convert electrons into another product of value.
“The main ingredient is plastic waste that would otherwise have an unsustainable afterlife, and the synthesis we use, where the chemical transformation takes place, is gentler than other materials for CO2 capture because we can make the synthesis in ambient temperatures. It also has the advantage that the technology can be scaled up more easily,” Margarita Poderyte says.
No conflict with recycling
During the development process, the researchers encountered concerns that their technology could undermine efforts to recycle plastic, which has been heavily invested in. That is not the case: it is complementary to recycling, not a competitor.
“In principle, we could use new plastic for our method, but our target is PET plastic that is difficult to recycle because of low quality, colouration or mixed sources – or that has decomposed to such a degree that it’s no longer suitable for recycling,” Margarita Poderyte says.
Co-author and Associate Professor at the Department of Chemistry, Jiwoong Lee, highlights the material’s flexibility.
“One of the impressive things about this material is that it stays effective for a long time. And flexible. It works efficiently from normal room temperature up to about 150 degrees Celsius. With this kind of tolerance to high temperatures, the material can be used at the end of industrial plants where the exhausts are typically hot,” Jiwoong Lee says.
From laboratory to innovation at the end of the chimney
With a potentially revolutionary idea, a proven method and an effective finished product, the researchers are now ready for the next step.
“We see great potential for this material, not just in the lab, but in real-life industrial carbon capture plants. The next big step is scaling up to produce the material in tonnes, and we’re already working to attract investments and make our invention a financially sustainable business venture,” Margarita Poderyte says.
The technical challenges do not worry the researchers. Instead, the decisive challenge, they say, is to persuade decision-makers to make the necessary investments. If they succeed in that, the invention could ultimately lead to significant changes.
Economic incentive to cleanse the oceans
The PET plastic that has been accumulating in the oceans, damaging ecosystems and breaking down into microplastics, is very well suited for the technology.
“If we can get our hands on the highly decomposed PET plastic floating in the world’s oceans, it will be a valuable resource for us as it’s so well suited for upcycling with our method,” Margarita Poderyte says.
“We’re not talking about stand-alone issues, nor will the solutions be. Our material can create a very concrete economic incentive to cleanse the oceans of plastic,” Jiwoong Lee says.
The research paper is published in Science Advances journal, which describes the chemical process behind the invention. The process is claimed to be gentle compared to existing technologies and, at the same time, well-suited for industrial scaling.

